
The Best Way to Manage Migraines Without Medication
For many people, migraines interfere with daily life, work and relationships. Understanding what’s behind them — and how to manage them naturally — can be a powerful step toward lasting relief.

Click Therapeutics’ migraine mobile app may now be used alongside standard drug therapies as a way to prevent episodic migraine in adults. In clinical trials, daily use of the app led to a statistically significant reduction in monthly migraine days.

In an interview, Munich Re Specialty Senior Vice President Jim Craig talked about the risk that accompanies innovation and the important role that insurers play.

Royalty Pharma’s milestone payment from the approval of Pfizer migraine drug Zavzpret stems from financing deals struck with the therapy's previous developer, Biohaven Pharmaceuticals. Royalty Pharma is also in line to receive royalty payments going beyond the next decade.

Pfizer is turning to M&A to get its next blockbuster, buying Biohaven six months after the two companies began a commercialization pact centered on the oral migraine drug Nurtec. The acquisition will lead to the spinout of Biohaven’s other assets into a new, publicly traded company capitalized with $275 million.

The European Commission approved Biohaven Pharmaceuticals migraine drug rimegepant. While the regulatory decision marks another milestone for Biohaven, it's also a win for Pfizer, which holds rights to commercialize the drug outside of the U.S.

Regulatory setbacks for a new migraine treatment have led Zosano Pharma to restructure, laying off about 31% of its staff. Zosano is one of several biotechs that announced cost-saving measures this week.

Biohaven Pharmaceuticals has seen strong U.S. market uptake for its oral migraine drug, Nurtec. With regulatory decisions looming around the world, the company has landed the marketing muscle of Pfizer, which is paying $500 million up front for the right to market that drug and another clinical-stage compound outside of the U.S.

AbbVie drug Quilpta, developed specifically for migraine prevention, is now FDA approved, the latest product in a new class of migraine medicines. The AbbVie drug is the second oral migraine prevention drug that the FDA has approved this year.
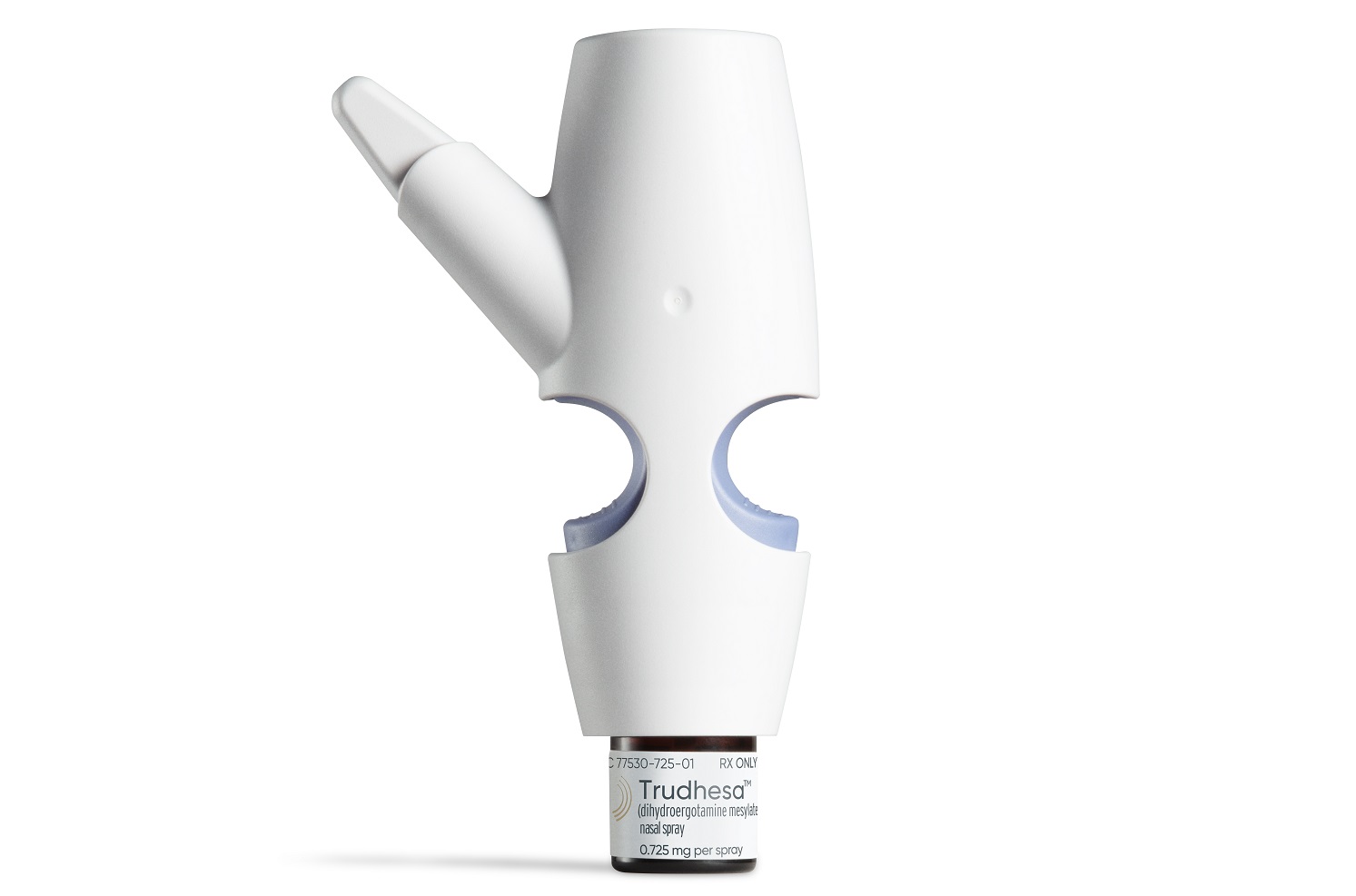
The FDA approved an Impel NeuroPharma migraine treatment, a nasal spray intended to bring pain relief more quickly than drugs administered in other ways. It marks the first approval for the Seattle company, which has developed a proprietary nasal delivery technology.

Satsuma Pharmaceuticals executives say they now understand why their intranasal migraine treatment failed a pivotal test. But even with a new Phase 3 plan, the company is behind a competitor that could launch its intranasal migraine treatment later this year.

Alder, based in a suburb of Seattle, has a monoclonal antibody that it has investigated as a treatment for migraine and is under FDA review, with the agency expected to decide on its approval in February of next year. Shares of Alder were up 83 percent on the news.

Eli Lilly announced Wednesday that it had acquired CoLucid Pharmaceuticals for $960M in an all-cash transaction, regaining ownership of lasmiditan, a late-stage migraine drug that could be commercialized as early as 2018.

The startup just raised $6 million to further its development of migraine-correcting spectacles.

There are only so many Advils a migraineur can pop before the ulcers and GI bleeds start setting in. They may be effective pain drugs, but NSAIDs are notorious for causing gastrointestinal distress – particularly when taken in the high doses needed to treat migraines. North Carolina specialty pharma startup Achelios Therapeutics has built a platform […]